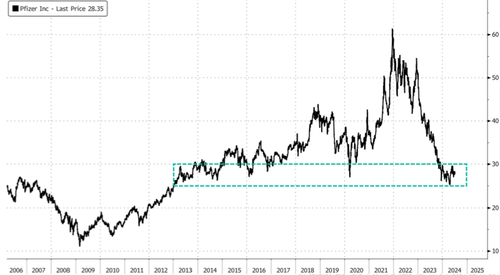
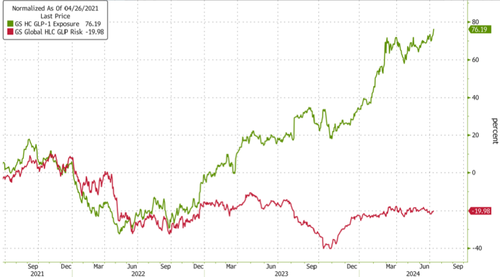
Shares of Pfizer Inc. are rising in premarket trading in New York following news that the drugmaker is progressing with its once-daily anti-obesity pill, 'danuglipron' in clinical trials.
"Pfizer plans to conduct dose optimization studies in the second half of 2024 evaluating multiple doses of the preferred modified release formulation to inform the registration enabling studies," the big pharma company wrote in a press release Thursday about its drug danuglipron, an oral glucagon-like peptide-1 (GLP-1) receptor agonist.
Here's further color on the danuglipron clinical:
-
Clinical evaluation of several modified release once-daily formulations of danuglipron resulted in encouraging pharmacokinetic data for several candidates with one showing the most favorable profile
-
The company plans to conduct dose optimization studies with a focus on the preferred formulation to inform the registration enabling studies
In December, Pfizer discontinued a twice-daily version of danuglipron after patients experienced tolerance issues. The company indicated that phase one trial data on the once-daily version would "inform a path forward."
"Obesity is a key therapeutic area for Pfizer, and the company has a robust pipeline of three clinical and several pre-clinical candidates. The most advanced of them, danuglipron, has demonstrated good efficacy in a twice-daily formulation, and we believe a once-daily formulation has the potential to have a competitive profile in the oral GLP-1 space," Mikael Dolsten, MD., PhD., Chief Scientific Officer & President, Pfizer Research and Development, wrote in a statement.
Dolsten continued, "Following a thorough analysis of our previous Phase 2b data and trial design, we believe that with the preferred modified release formulation and future trial design optimization, we can advance a competitive oral GLP-1 molecule into registration enabling studies, with the goal of addressing the present and persistent medical needs of people living with obesity."
In markets, shares of Pfizer are up 3% to around the $29 handle. Shares have been halved in recent years, falling to early 2013 lows, following the end of the virus pandemic that sent Covid vaccine demand plunging.
Companies with exposure to GLP-1s are continuing to march higher.
Pfizer is a handful of drugmakers trying to carve out a slice of the GLP-1 market to tackle obesity in the West. Wall Street analysts have projected the industry could be worth $100 billion by the end of the decade.
In June, CEO Albert Bourla said GLP-1s are only "scratching the surface of what we will see in obesity."
Instead of GLP-1s, perhaps consumers should try intermittent fasting, eating healthy, and daily exercise.
Meanwhile, "New Study Reveals Ozempic, Wegovy Linked To' Potentially Blinding Eye Condition'"...