On Wednesday, the Food and Drug Administration announced that the supply shortages of Eli Lilly's popular weight-loss and diabetes drugs have been resolved. These drugs have been in short supply since the anti-obesity drug craze erupted several years ago. Now, an ample supply of GLP-1 drugs could signal that the 'fat bubble' in equity markets has popped.
FDA said that Lilly's Zepbound and Mounjaro multi-year shortage is over, adding that there are "legal restrictions on making copies of FDA-approved drugs" when there isn't a shortage.
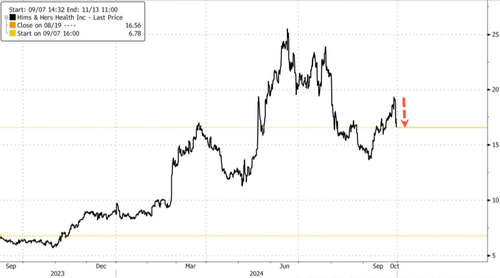
Shares of telehealth firm Hims & Hers Health plunged nearly 12% in premarket trading in New York. The company sells copycat weight-loss drugs. As noted above on X, "The Fat bubble has officially popped."
Lilly's shares in premarket trading were flat to slightly higher, while Novo Nordisk, the maker of Wegovy, was half a percent higher in Denmark.
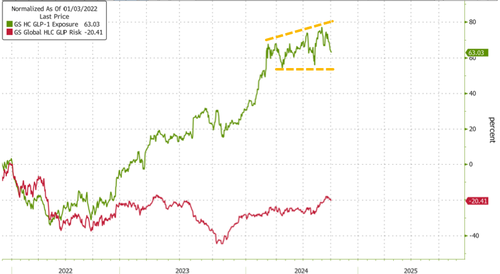
Using Goldman's index of companies with high exposure to GLP-1s, momentum has stalled throughout 2024 as companies at risk from GLP-1s' success have been clawing back losses.
Eli Lilly Executive Vice President Patrik Jonsson released a statement saying that Lilly has "invested significantly" to expand the manufacturing of GLP-1 drugs and bring new options to the market. WaPo quoted him as saying the reversal in the shortage reflects Lilly's "commitment to providing a steady stream of genuine and safe medicines."
Lilly and its competitor, Novo Nordisk, which makes the diabetes drug Ozempic and weight loss magic drug Wegovy, have been locked in a vicious battle against the copycat GLP-1s market. Lawyers representing Lilly issued warnings in August to prescribers to cease selling the copycat drugs, WaPo noted.
Bloomberg cited experts that estimate hundreds of thousands of Americans have used copycat versions of Lilly and Novo weight loss drugs, adding, "The makers of such compounded drugs are bringing in as much as $1 billion a year, according to investment bankers who work with the industry."
Scott Brunner, CEO of the trade group Alliance for Pharmacy Compounding, told WaPo that the FDA's ending the shortage designation for those drugs could be difficult for consumers who have taken off-brand compounded tripeptides.
"They are being cut off cold turkey, and their prescription is no longer fillable," Brunner said, noting that pharmacies "must immediately cease preparing and dispensing compounded copies of Mounjaro and Zepbound."