Novo Nordisk shares gained in Copenhagen trading on Monday after the U.S. FDA approved Wegovy for the treatment of metabolic-associated steatohepatitis (MASH), a severe form of liver disease. The approval gives Novo a first-mover advantage over Eli Lilly in the U.S. market.
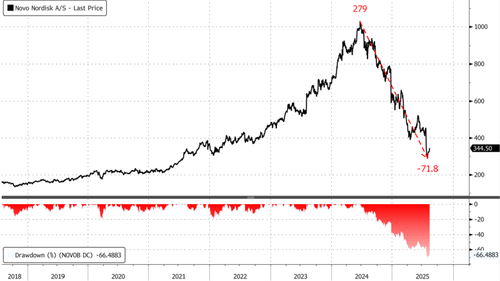
Year-to-date, Novo shares have lost nearly 50% amid intensifying GLP-1 competition, including Eli Lilly's obesity drug Zepbound and copycat threats (GLP-1 compounds) from companies such as telehealth company Hims & Hers Health. The MASH approval offers a potential incremental growth driver and could reverse some of the negative sentiment surrounding the stock this year. This raises the question for investors whether current stock levels represent a buy-the-dip opportunity.
The FDA's decision marks the second positive news for Novo this month, following Lilly's oral obesity pill, orforglipron, that met its primary endpoints but fell short of Wall Street's expectations. At the time, shares of Lilly plunged in the U.S., the most since the Dot Com bust, while Novo shares rose.
Wegovy's approval for MASH puts Novo ahead of Lilly, which is still preparing trials for Zepbound and its next-generation drug, retatrutide.
Commentary from Wall Street analysts:
BMO Capital Markets (Market Perform, Evan Seigerman):
FDA approval of Wegovy for MASH could help shift sentiment after a tough start to the year marked by compounder competition and guidance cuts.
Sees potential for $1.9B in peak global unadjusted revenues from the MASH indication, with secondary uses like this expected to broaden Wegovy's coverage over time.
Maintains cautious stance, calling Novo a "show-me story" into 2H25. Key catalysts to watch: Wegovy's positioning with CVS and the expected 4Q decision on 25mg oral semaglutide for obesity.
UBS (Neutral, Matthew Weston):
- Positive. 3Q timing known but earlier than expected. Label in line F2-F3 MASH. Adds growth driver & temporary differentiation vs tirzepatide (UBSe MASH label 2027 launch). NPV: $500m US Wegovy MASH peak, 0.7% NPV ($1b peak Globally, 1.2% NPV).
Wegovy being cleared for MASH is a step in the right direction for investors after suffering a 45% decline on the year. Shares in Copenhagen on Monday are higher by about 6%.
For Novo shares to rebound meaningfully, the company must address compounded GLP-1 knockoffs that have flooded the U.S. market, undermining demand and slowing Wegovy's expansion. Novo noted in its earnings release earlier this month that it is "pursuing multiple strategies, including litigation, to protect patients from knockoff 'semaglutide' drugs." Meanwhile, the clock is ticking for HIMS.
Loading recommendations...